Why Torque Testing Is Critical in Catheter Development
Catheters play a vital role in minimally invasive procedures, where precision and control are paramount. Their design must balance flexibility, durability, and sensitivity to ensure safe navigation through complex vascular pathways.
Torqueability refers to the catheter’s ability to transmit rotational force from the proximal end to the distal tip. During testing, the catheter is inserted into an anatomical model, such as those specified in ASTM F2394 protocols. A torque is applied at the proximal end while the rotational response at the distal end is evaluated. Low torqueability can lead to wire tip whipping, reduced control, or device entrapment and breakage within lesions. These issues pose serious procedural risks, making torqueability a critical performance metric.
In addition to torqueability, torque-to-failure testing evaluates the maximum twisting force a catheter can withstand before structural failure. This assessment ensures the device maintains mechanical integrity under real-world conditions.
Optimizing Catheter Design with FUTEK’s Rotary Torque Sensors
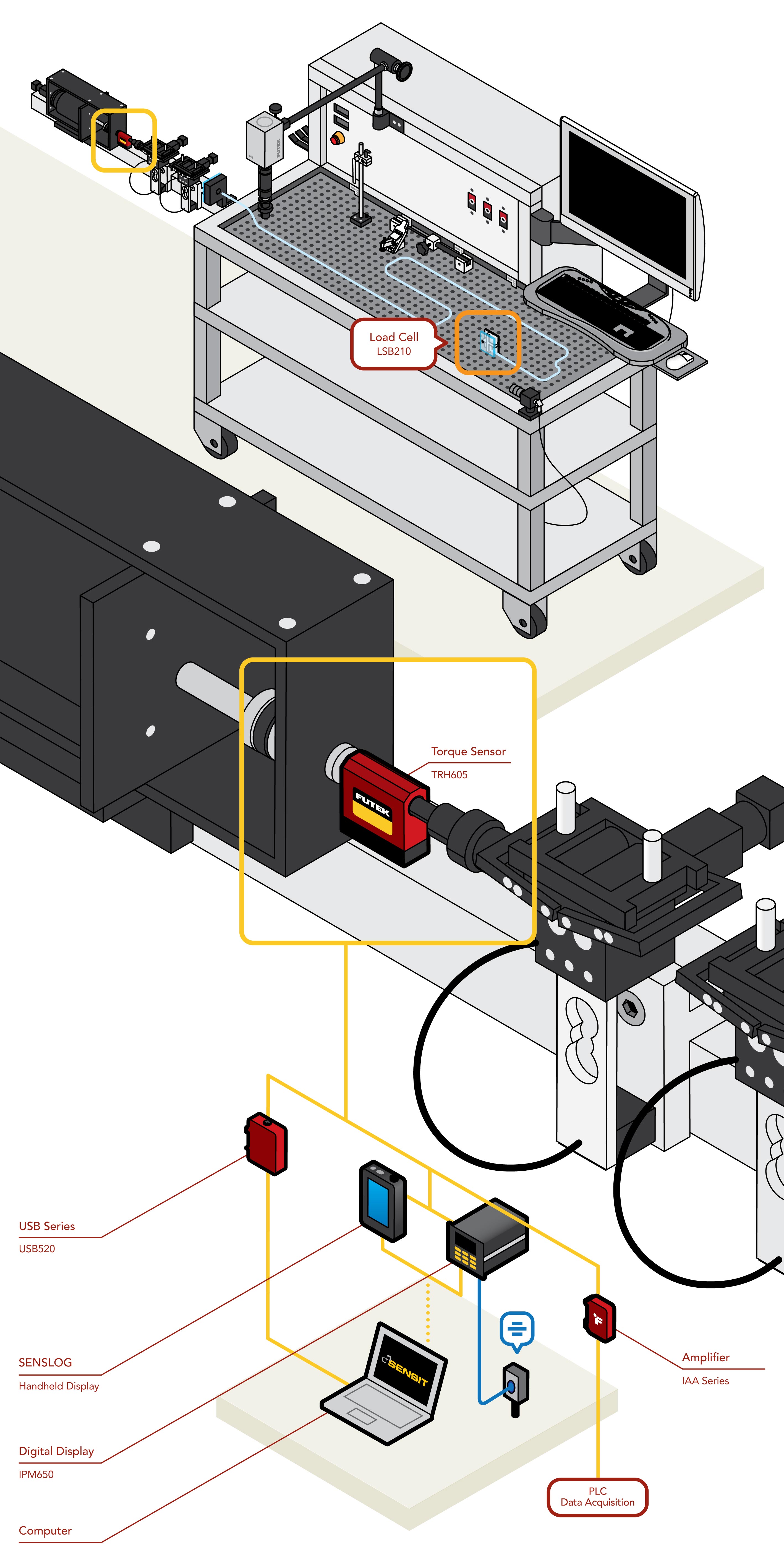
Catheter testing requires high precision equipment to ensure compliance with standards such as ISO 25539. FUTEK’s TRH605 Rotary Torque Sensor is engineered to meet the demands of catheter testing, featuring brushless, non-contact technology for improved longevity compared to traditional contact sensors. With an accuracy of 0.2% of Rated Output (RO) and torque capacities starting at 0.5 Nm, the TRH605 is an ideal choice for torqueability and torque-to-failure evaluations. A built-in encoder provides a ±5 VDC output along with TTL pulse signals for angle and RPM measurements. These features make the TRH605 a versatile tool for capturing multiple kinds of torque data.
When post-processing the data is necessary, the TRH605 can be paired with FUTEK’s USB520 for seamless integration with SENSITTM Test and Measurement Software. Data can be visualized, logged, and graphed with sampling rates of up to 4800 samples per second. For portable applications, the IHH505 SensLogTM Handheld Display and IPM650 Panel Mount Display offer convenient options for immediate torque visualization and verification.
Holistic Testing Through Load Cell Integration
In addition to torque, pushability and trackability forces are also essential in evaluating catheter performance. FUTEK’s testing solutions can be expanded to include load cells, enabling simultaneous measurement of force and torque. Visit Catheter Track Force Test to learn how adding a FUTEK load cell can create a comprehensive catheter force and torque sensing solution.
How it Works
A TRH605 Rotary Torque Sensor is installed in-line with a driving motor to quantify the rotary force exerted to advance the catheter through a tortuous anatomical model (test tube).
As the catheter is rotated and advanced, torques applied at the proximal end are recorded to assess torqueability.
These measurements can be streamed to a computer utilizing FUTEK's USB520. Alternatively, the IHH505 SensLogTM Handheld Display or IPM650 Panel Mount Display can provide immediate data-visualization, with the ability to connect to a PC.
Data can be monitored with FUTEK's SENSIT™ Test and Measurement Software, or through a custom-built program utilizing provided examples and DLL files.
Products in Use
FUTEK’s Rotary Torque Sensor – Hex-Drive (TRH605) paired with USB Solutions and SENSIT™ Test and Measurement Software
Contact Us
Please Contact Us with questions.
Why Torque Testing Is Critical in Catheter Development
Catheters play a vital role in minimally invasive procedures, where precision and control are paramount. Their design must balance flexibility, durability, and sensitivity to ensure safe navigation through complex vascular pathways.
Torqueability refers to the catheter’s ability to transmit rotational force from the proximal end to the distal tip. During testing, the catheter is inserted into an anatomical model, such as those specified in ASTM F2394 protocols. A torque is applied at the proximal end while the rotational response at the distal end is evaluated. Low torqueability can lead to wire tip whipping, reduced control, or device entrapment and breakage within lesions. These issues pose serious procedural risks, making torqueability a critical performance metric.
In addition to torqueability, torque-to-failure testing evaluates the maximum twisting force a catheter can withstand before structural failure. This assessment ensures the device maintains mechanical integrity under real-world conditions.
Optimizing Catheter Design with FUTEK’s Rotary Torque Sensors
Catheter testing requires high precision equipment to ensure compliance with standards such as ISO 25539. FUTEK’s TRH605 Rotary Torque Sensor is engineered to meet the demands of catheter testing, featuring brushless, non-contact technology for improved longevity compared to traditional contact sensors. With an accuracy of 0.2% of Rated Output (RO) and torque capacities starting at 0.5 Nm, the TRH605 is an ideal choice for torqueability and torque-to-failure evaluations. A built-in encoder provides a ±5 VDC output along with TTL pulse signals for angle and RPM measurements. These features make the TRH605 a versatile tool for capturing multiple kinds of torque data.
When post-processing the data is necessary, the TRH605 can be paired with FUTEK’s USB520 for seamless integration with SENSITTM Test and Measurement Software. Data can be visualized, logged, and graphed with sampling rates of up to 4800 samples per second. For portable applications, the IHH505 SensLogTM Handheld Display and IPM650 Panel Mount Display offer convenient options for immediate torque visualization and verification.
Holistic Testing Through Load Cell Integration
In addition to torque, pushability and trackability forces are also essential in evaluating catheter performance. FUTEK’s testing solutions can be expanded to include load cells, enabling simultaneous measurement of force and torque. Visit Catheter Track Force Test to learn how adding a FUTEK load cell can create a comprehensive catheter force and torque sensing solution.