Haptic Feedback in Robot Assisted Minimally Invasive Surgery
Challenges in Designing and Manufacturing Haptic Sensors for Robotic Surgery Platforms
The design and manufacture of a haptic feedback sensor for a robotic surgery platform entail two distinct but complementary aspects: overcoming technical obstacles of designing a sensor for intra- abdominal force measurement (miniaturization, biocompatibility, autoclavability, and high reusability), while maintaining the minimally required specification of a high-grade force measurement solution (nonlinearity, hysteresis, repeatability, cross-talk, bandwidth and noise-free resolution). Furthermore, the sensor solution provider must have a talented and multidisciplinary engineering team, in-house manufacturing capabilities supported by fully developed quality processes and product/project management proficiency to handle the complex, resource-limited and multi-stakeholder new product development environment.
Historical Perspective of Surgery Practice
Surgery is a centuries-old healing practice whose etymology comes from the Greek word cheirourgia (performed by the hands). Surgical procedures evolved over the centuries concerning instruments, pain control, infection prevention and, most importantly, surgical techniques, resulting in enhanced medical treatment and ultimately extending human lifespan.
Minimally invasive surgery (MIS) is a modern technique that allows interventionists to perform operations through small incisions (usually 5 – 15 mm). Besides its clear advantages, MIS is
more difficult to perform compared to traditional procedures. Other inherent drawbacks are (a) limited motion due to straight laparoscopic instruments and fixation enforced by the small incision in the abdominal wall; (b) impaired vision, due the two-dimensional imaging; (c) usage of long instruments amplifies the effects of surgeon’s tremor; (d) poor ergonomics imposed to the surgeon; and (e) loss of haptic feedback, which is distorted by friction forces on the instrument and reactionary forces from the abdominal wall (Ballantine, 2002).
Gains and Losses of Minimally Invasive Robotic Surgery
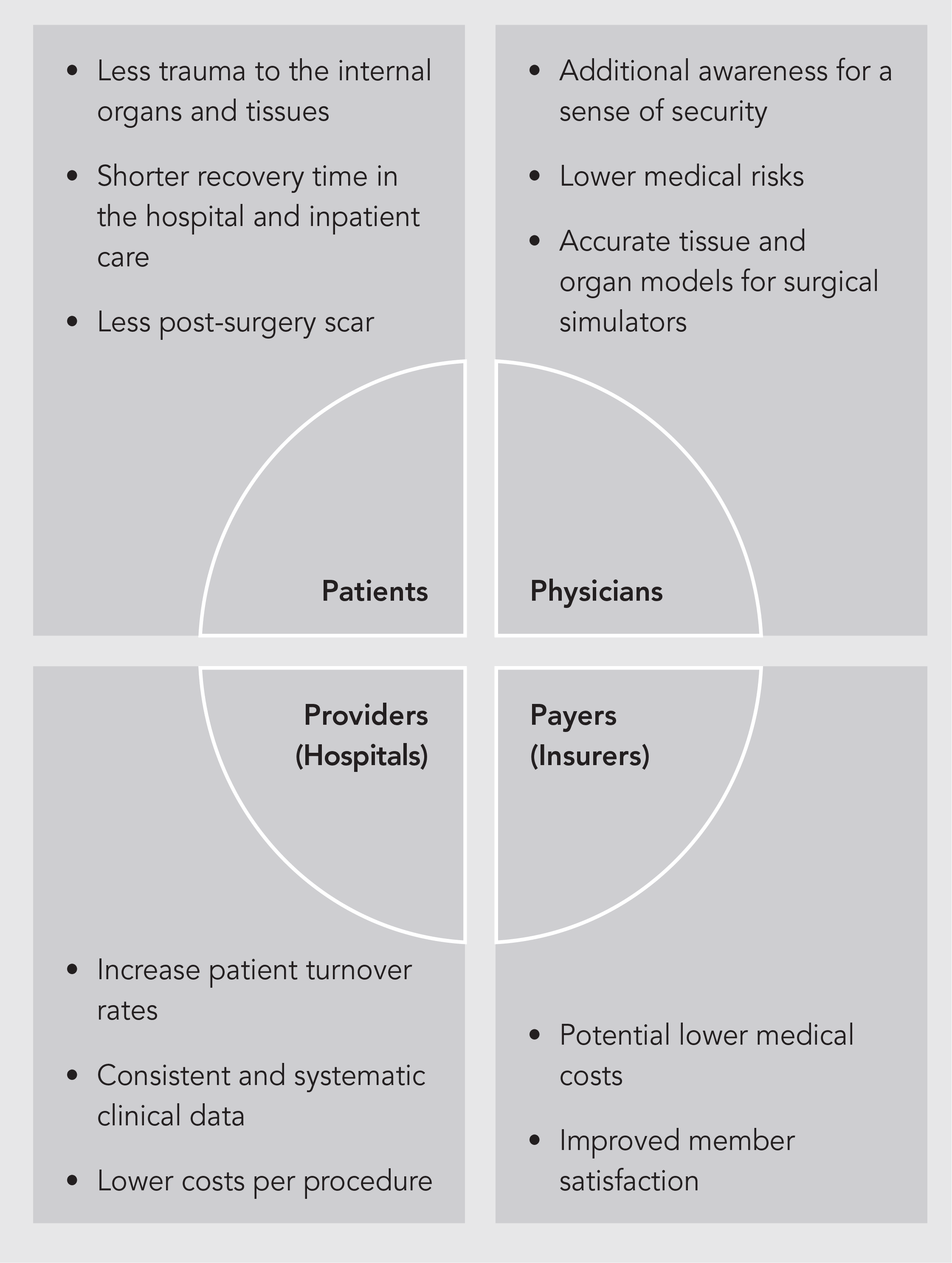
Minimally Invasive Robotic Surgery (MIRS) offers solutions to either minimize or eliminate many of the pitfalls associated with traditional laparoscopic surgery. Current available MIRS platforms such as the Da Vinci Surgical System, approved by the U.S. Food and Drug Administration in 2000, was a historical milestone of surgical treatments. The ability to leverage laparoscopic surgery advantages while augmenting surgeons’ dexterity and visualization and eliminating the ergonomic discomfort of long surgeries, makes MIRS undoubtedly an essential technology for the patient, surgeons, and hospitals.
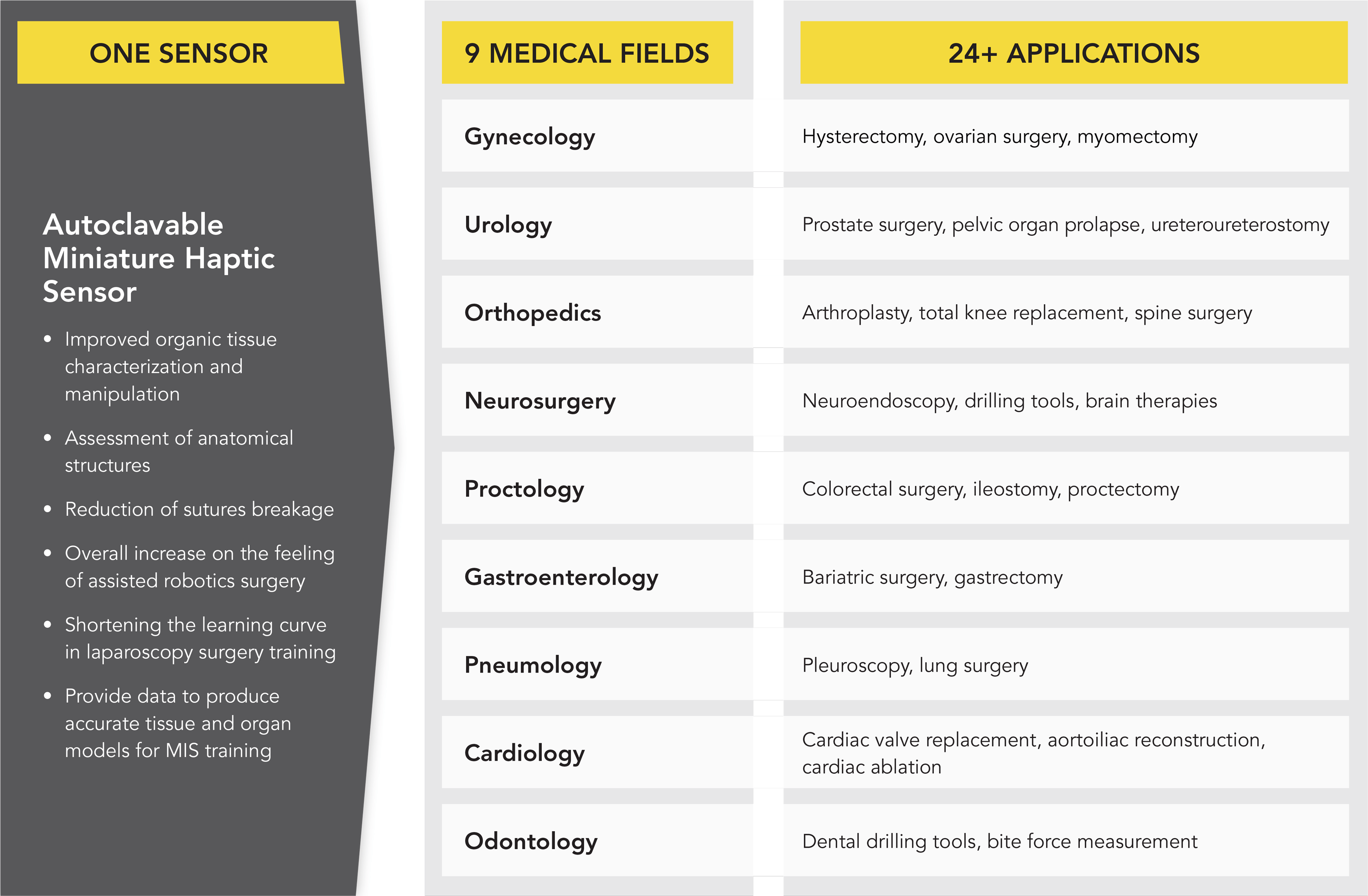
However, despite all improvements brought by currently commercially available MIRS, haptic feedback in robot assisted minimally invasive surgery is still a major limitation reported by robot-assisted surgeons. Since the interventionist no longer manipulates the instrument directly, the natural haptic feedback is eliminated. Haptics is a conjunction of both kinesthetic (form and shape of muscles, tissues and joints) as well as tactile (cutaneous texture and fine detail) perception, and is a combination of many physical variables such as force, distributed pressure, temperature, and vibration (Okamura, 2009). Direct benefits of sensing interaction forces at the surgical end-effector are (a) improved organic tissue characterization and manipulation, (b) assessment of anatomical structures, (c) reduction of sutures breakage, and (d) overall increase in the feeling of assisted robotics surgery (Callaghan et al., 2008).
Haptic feedback in robot assisted minimally invasive surgery also plays a fundamental role in shortening the learning curve for young surgeons in MIRS training. A tertiary benefit of accurate real-time direct force measurement is that the data collected from these sensors can be utilized to produce accurate tissue and organ models for surgical simulators used in MIS training.
Technical and Economic Challenges
Adding to the inherent complexity of measuring haptics, engineers and neuroscientists also face important issues that require consideration prior to the sensor design and manufacturing stages. The location of the sensing element, which significantly influences the measurement consistency, presents MIRS designers with a dilemma: should they place the sensor outside the abdomen wall near the actuation mechanism driving the end-effector (a.k.a. Indirect Force Sensing), or inside the patient at the instrument tip, embedded on the end-effector (a.k.a. Direct Force Sensing).
| Table 1: Comparison between Direct vs. Indirect Force Measurement | ||
|---|---|---|
| Indirect Sensing | Direct Sensing | |
| Measurement Accuracy | Tissue contact forces are masked by friction between the instrument trocar and the insertion port. Measurement distortion is also caused by mechanical linkages connecting the end-effector and the actuator (strings) and gravitational effects of mass along the instrument axis. This method does not consider gripping forces at the end-effector. |
Frictions and transmission disturbance forces do not affect the haptics feedback measurement once the sensor is located near the end-effector. This approach allows the measurement of gripping forces at the jaw of the end-effector. |
| Space Requirements | The sensing element would not need to pass thru the insertion port (abdominal wall), meaning there are not many dimensional and material constraints. | Abdominal laparoscopic insertion ports range from 5 to 15 mm, which severely limits the sensor to only ~12 mm in diameter. |
| Sterilization and Biocompatibility | Components that lie outside the abdominal cavity would not need to be sterilized. | Must follow standard sterilization used for surgical instruments. Sterilization protocol in an autoclave determines 121 °C and 205 kPa steam for 4 to 15 minutes. Bonding agents, microelectronic components, strain gages, and electrical conductors must be able to withstand these temperatures and pressures. |
The pros and cons of these two approaches are associated with measurement accuracy, size restrictions and sterilization and biocompatibility requirements (Gupta, 2011). Table 1 compares these two force measurement methods.
In the MIRS applications where very delicate instrument-tissue interaction forces need to give precise feedback to the surgeon, measurement accuracy is sine qua non, which makes intra-abdominal direct sensing the ideal option.
However, this novel approach not only brings the design and manufacturing challenges described in Table 1 but also demands higher reusability. Commercially available MIRS systems that are modular in design allow the laparoscopic instrument to be reutilized approximately 12 to 20 times. Adding the sensing element near to the end-effector invariably increases the cost of the instrument and demands further consideration during the design stage in order to enhance sensor reusability. Appropriate electronic components, strain measurement method and electrical connections have to withstand additional autoclavable cycles as well as survive a high PH washing. Coping with these special design requirements invariably increases the unitary cost per sensor. However, extended lifespan and number of cycles consequently reduce the cost per cycle and bring financial affordability to direct measurement method (Callaghan, 2008).
Hermeticity of high precision sub-miniature load sensing elements is equally challenging to intra- abdominal direct force measurement. The conventional approach to sealing electronic components is the adoption of conformal coatings, which are extensively utilized in submersible devices. As much as this solution provides protection in low-pressure water submersion environments for consumer electronics, coating protection is not sufficiently airtight and is not suitable for high-reliability medical, reusable and sterilizable solutions. Under extreme process controls conformal coatings have shown to be marginal and provide upwards of 20 to 30 autoclave cycles. The autoclave sterilization process presents a harsher physicochemical environment utilizing high pressure and high temperature saturated steam. Similar to helium leak detection technology, saturated steam particles are much smaller in size compared to water particles and are capable of penetrating and degrading the coating over time causing the device to fail in a hardly predictable manner. An alternative and conventional approach to achieving hermeticity is to weld on a header interface to the sensor. Again, welding faces obstacles in miniaturized sensors due to its size constraints. All in all, a novel and robust approach is a monolithic sensor using custom formulated, Ct matched, chemically neutral, high temperature fused isolator technology used to feed electrical conductors through the walls of the hermetically sealed active sensing element. The fused isolator technology has shown reliability in the hundreds to thousands of autoclave cycles.
Other Design Considerations
As aforementioned, miniaturization, biocompatibility, autoclavability and high reusability are some of the unique characteristics imposed to a haptic sensor by the surgical environment. In addition to that, it is imperative that designers also meet requirements that are inherent to any high-performance force measurement device.
Extraneous loads (or mechanical crosstalk) compensation, provides optimal resistance to off-axis loads to assure maximum operating life and minimize reading errors. Force and torque sensors are engineered to capture forces along the Cartesian axes, typically X, Y and Z. From these three orthogonal axes, one to six measurement channels derives three force channels (Fx, Fy and Fz) and three torque or moment channels (Mx, My and Mz). Theoretically, a load applied along one of the axes should not produce a measurement in any of the other channels, but this is not always the case. For a majority of force sensors, this undesired cross-channel interference will be between 1 and 5% and, considering that one channel can capture extraneous loads from five other channels, the total crosstalk could be as high as 5 to 25%.
In robotic surgery, the sensor must be designed to negate the extraneous or cross-talk loads which include frictions between the end-effector instrument and trocar, reactionary forces from the abdominal wall and gravitational effect of mass along the instrument axis. In some occasions, miniaturized sensors are very limited in space and have to compensate side loads using alternate methods such as electronic or algorithmic compensation.
Calibration of direct inline force sensor imposes restrictions as well. The calibration fixtures are optimized with SR buttons to direct load precisely through the sensor of the part. If the calibration assembly is not equipped with such arrangements, the final calibration might be affected by parallel load paths.
Thermal effect is also a major challenge in strain measurement. Temperature variations cause material expansion, gage factor coefficient variation and other undesirable effects on the measurement result. For this reason, temperature compensation is paramount to ensure accuracy and long-term stability even when exposed to severe ambient temperature oscillations. The measures to counteract temperature effects on the readings are (a) the use of high-quality, custom and self-compensated strain gages compatible with the thermal expansion coefficient of the sensing element material; (b) use of half or full Wheatstone bridge circuit configuration installed in both load directions (tension and compression) to correct for temperature drift and (c) fully internally temperature compensation of zero balance and output range without the necessity of external conditioning circuitry.
In some special cases, the use of custom strain gages with reduced solder connections helps reduce temperature impacts from solder joints. Usually, a regular force sensor with four individual strain gages has upwards of 16 solder joints, while custom strain elements can reduce this down to less than six. This design consideration improves reliability as the solder joint, as an opportunity for failure, is significantly reduced.
During the design phase it is also imperative to consider such sensors to meet high reliability along with high-volume manufacturability, taking into consideration the equipment and processes that will be required should a device be designated for high-volume manufacturing. After all, the automated, high-volume processes could be slightly or significantly different than the benchtop or prototype equipment used for producing lower volumes. The scalability must maintain focus on reducing failure points during the manufacturing process, along with failure points that could occur on the field.
Testing & Measurement for medical applications is more related to the ability of a measurement device that can withstand a high number of cycles rather than resist to strenuous structural stress. In particular for medical sensors, the overload and fatigue testing must be performed in conjunction with the sterilization testing in an intercalated process with several cycles of fatigue and sterilization testing. The ability to survive hundreds of overload cycles while maintaining hermeticity translates into a failure-free, high-reliability sensor with lower MTBF and more competitive total cost of ownership.
Product Development Goes Beyond Sensor Design Challenges
Although understanding the inherent design challenges of the haptic autoclavable sensor is imperative, the sensor solution provider has to be equipped with a talented multidisciplinary engineering team, in-house manufacturing capabilities supported by fully developed quality processes and product/project management proficiency to handle the complex, resource-limited, and fast-paced new product development environment.
A multi-discipline engineering team identifies and translates customer needs into a cross-functional design as well as deploys novel approaches to old problems whose solutions are not easily found by a single, one-sided technical view. From this perspective, a design team must be compounded by mechanical engineers and able to navigate through the latest machine design technologies such as in-depth finite element analysis (FEA), 3D printing and rapid prototyping. The team should also include electrical engineers capable of designing instrumentation solutions to meet strict signal conditioning requirements. This multidisciplinary approach results in a sensor element that meets the specifications in terms of nonlinearity, hysteresis, repeatability and cross-talk, as well as an electronic instrument that delivers analog and digital output, high sampling rate and bandwidth, high noise-free resolution and low power consumption, both equally necessary for a reliable turnkey haptics measurement solution.
When it comes to the production floor, a comprehensive in-house manufacturing capability enables the sensor design company to control all aspects of the production process to better meet the demanding schedule and quality requirements. Moreover, strategic control of all manufacturing processes (machining, lamination, wiring, calibration), allows the design company to engineer sensors with a Design for Manufacturability (DFM) mentality. This strategic control of manufacturing boils down to methodically selecting the bill of material, defining the testing plans, complying with standards and protocols and ultimately strategizing the manufacturing phase based on economic constraints.
Quality is expensive, but lack of quality costs even more. In medical applications where failure is not an option and tolerances are tight, the project stakeholders cannot afford to have quality as a meager department of testers whose job is to find defects before the customer does, but rather a rigorous and relentless commitment to a culture of excellence which permeates throughout all company processes, from design to shipping. Needless to say that the sensor solution provider must demonstrate its quality system is effectively implemented and maintained thru ISO 13485 certification as well ISO 17025 accredited testing and calibration laboratories.
Time to market and cost control is paramount when developing a medical device, and it also holds true for a haptic feedback sensor, considering the latter as part of the complex surgical robot supply chain. With that in mind, the company product managers must be fluent in agile/waterfall project management practices to integrate and streamline project activities, track controllable costs and, most importantly, ensure that information flows among multiple stakeholders promptly.
Conclusion
Surgical robotic companies are pursuing a marketable product that incorporates Haptic feedback in robot assisted minimally invasive surgery whereas few specialized sensor technology companies combine the competencies to offer a measurement solution that meets all design requirements imposed by intra-abdominal force measurement in MIRS: miniaturization, biocompatibility, autoclavability, high reusability and measurement accuracy.
References
- Haptic feedback in robot-assisted minimally invasive surgery Allison Okamura - Current Opinion in Urology – 2009.
- The Pitfalls of Laparoscopic Surgery: Challenges for Robotics and Telerobotic Surgery Garth Ballantyne - Surgical Laparoscopy, Endoscopy & Percutaneous Techniques – 2002.
- Force Measurement Methods in Telerobotic Surgery: Implications for End-Effector Manufacture Dean Callaghan, et al - Dublin Institute of Technology – 2008.
- Application of Biomedical Engineering in Force and Tactile Sensing for Robotic MIS Sweta Gupta – University of California Irvine – 2011.